So3 2 Lewis Structure
Download Free PDF View PDF. To use the Lewis Structure Calculator follow these steps.

So3 2 Lewis Structure Sulfite Ion Lewis Molecules Understanding
Download Free PDF View PDF.

. The Lewis dot structure isnt applicable with hydrocarbons and molecules consisting of two atoms of the same element. Molecules with less than eight electrons. The Lewis structure is a The Lewis structure is a Q.
Molecules have an odd number of electrons eg NO. Download Free PDF View PDF. Molecules in one or more atoms have more than eight electrons eg SF6.
Enter the formula of the molecule in the field provided for it. An example is the BF3. Organic Chemistry 2 edition by Jonathan Clayden Nick Greeves and Stuart Warren.
For example if we want to obtain the Lewis structure of the Sulfate ion SO 4 2 we must first enter the charge by typing -2 or by entering -2 in the charge field and pressing the Add button. Lewiss bonding theory is based on the octet rule. The Art of Writing Reasonable Organic Reaction Mechanisms.
Then we write the rest of the formula being as follows. Lewis structure isnt suitable if. Draw the Lewis structure of CH32NH a neutral compound.
Solution for In the correct Lewis Electron-Dot Formula for SF4 molecule the number of nonbonding electrons on the central sulfur atom is.

Lewis Structure Of So3 Sulfur Trioxide Exceptions To The Octet Rule So Tricky Lewis Structure Can Be Chemistry Education Octet Rule Free Science Lesson

So3 Molecular Geometry Bond Angles Sulfur Trioxide Molecular Geometry Molecular Molecules

So3 2 Lewis Structure Sulfite Ion Lewis Molecules Understanding
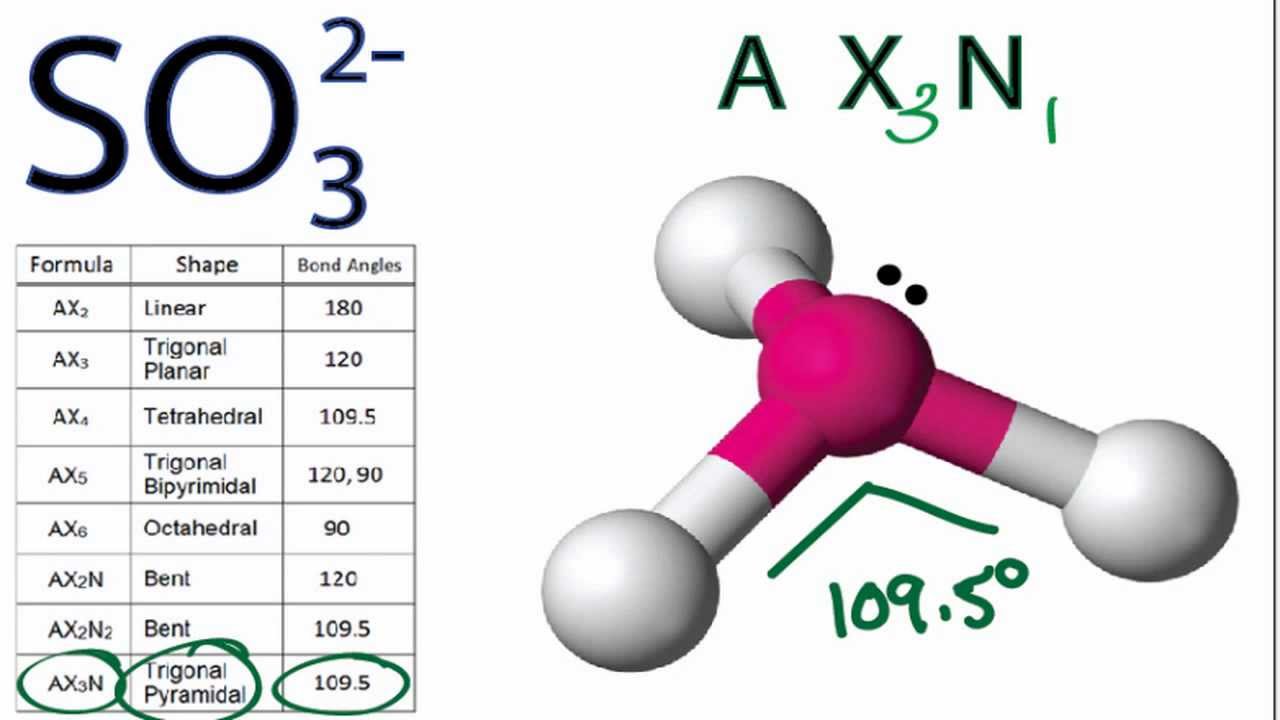
So32 Molecular Geometry Shape And Bond Angles Molecular Geometry Chemistry Help Molecular
0 Response to "So3 2 Lewis Structure"
Post a Comment